|
|
 |
|
|
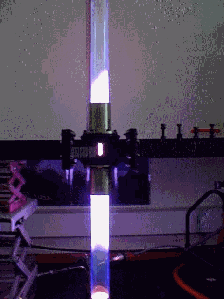
Microwave chemistryMicrowave Chemistry is becoming increasingly popular in synthetic chemistry. Microwave chemistry research literature has literally grown exponentially since the technique took off in the mid 1980’s. Debates still continue about precisely why microwaves are particularly effective in many reactions, but from a practical point of view, this is beside the point. The fact is that microwave heating can do a number of things which conventional heating cannot. First of all microwave heating is direct - energy is absorbed by the soley by the sample, and is not wasted on heating the sample vessel. Direct heating also means that it is a highly controllable form of heating: lag-times in heating regimes are very small, making rapid changes in temperature possible. This makes microwave heating highly efficient, and in many cases, this efficiency will over-ride the fact that microwave energy is relatively expensive. The direct nature of microwave heating makes it possible to heat specific components of a reaction in preference to others - in many cases, it is possible to ‘focus’ the energy on specific geometric regions of the sample - allowing reactions to be performed under highly inhomogenous conditions. Our work in Edinburgh is directed towards expanding the variety of microwave techniques which are available. At present, microwave reactors exist which are capable of heating samples at high pressure, but at relatively modest temperatures (100Bar, <250C), or which are capable of heating samples to very high temperatures at much lower pressures. We have developed a method for introducing microwaves into a very high pressure cavity and we are currently designing and building a high pressure,high temperature microwave applicator capable of operating at >100Bar and with sample temperatures in excess of 1000C. Several designs are currently under investigation for samples of either 500cm3 or approximately 5cm3, based on either cylindrical waveguide or coaxial applicators. A second field of investigation is in the area of particle preparation techniques. Particle preparation methods are interesting to a variety of industries such as pigments and ceramics. Particles may be prepared by a number of physical and chemical methods, and our approach is based on the method of aerosol pyrolysis. In this method, a solution containing precursor materials is converted into an aerosol and directed into a high temperature furnace. Particles are formed as the solvent is driven off to leave solid spheres of precursor compounds which subsequently react and condense - ideally yielding monodispersed, highly homogeneous spherical particles. Formation and reaction occurs very rapidly because the particle size is so small. Furnace techniques, however, suffer from inhomogenous temperature characteristics across the reaction volume, and this leads to inhomogeneity in the particles themselves. Our method employs a low pressure microwave induced plasma (MIP) to give a more efficient and homogeneous temperature cross section in the particle stream.
In recent years, we have explored the formation of iron oxide particles prepared by the hydrolysis of iron salts from aqueous solution. Curiously, microwave heating at sub-reflux temperatures yields needle-shaped magnetite particles, in contrast to the near-spherical particles which are produced by conventional heating methods. The precise reason for this is as yet unclear, but it seems unlikely that superheating is involved, since at no temperature is an analagous product found in the conventionally heated samples.
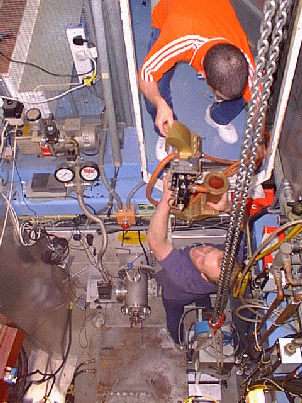
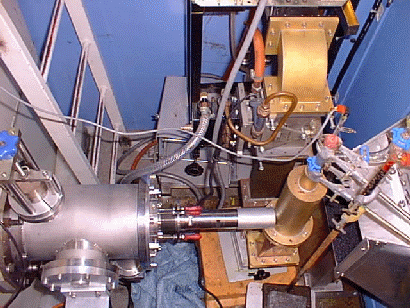
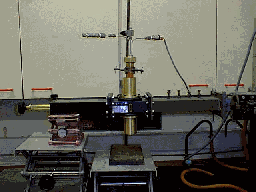
Installation (left) of the first in situ neutron diffraction microwave apparatus (Right) The versatility of microwave heating has allowed us to build other microwave applicators without disrupting the principal project aims. We currently run a low pressure microwave reactor which operates at a pressure of 0-15Bar. The pressure can be maintained to an accuracy of 0.01Bar for up to several days. Since the rate at which reactants are heated can make the difference between obtaining the thermodynamically favoured product and the first-formed kinetically favoured product. We are currently using this apparatus to to investigate reactions in which this effect may be important, with a view to optimising a number of organic and inorganic syntheses.
Microwave radiation is becoming a common source of energy in accelerating chemical reactions, particularly in aqueous solution. {advantage}. However, it suffers from a number of disadvantages. Monitoring and controlling temperature is made difficult because it is not possible to use conventional thermometers such as thermocouples; it is also difficult to contain reactions at extreme temperatures and pressures because metal may not used for vessels. We are building apparatus for high temperature, high pressure microwave chemistry that aims to bypass these problems by use of special microwave guides and ceramic components, and thus explore the potential of the technique more fully for solid-state ceramic synthesis. We are also developing techniques for high-power microwave plasma heating of aerosols to produce fine powders of a wide variety of solids with potential applications in magnetic recording and electronic devices. The use of a Raman spectrometer to monitor molecules and molecular fragments as they pass trough the plasma should enable us to understand and perhaps control the particle growth process. Reference
Return to Dr. Whittaker's Page This page was last updated on the 2nd November 1999 |
|