|
|
 |
||||||||||||||||||||||||||||||||||||||||||||
Microwave Heating and Intercalation Chemistry
© G. Whittaker, 1994 & 2007. This work, or extracts from this work may be reproduced only with the written permission of the author.
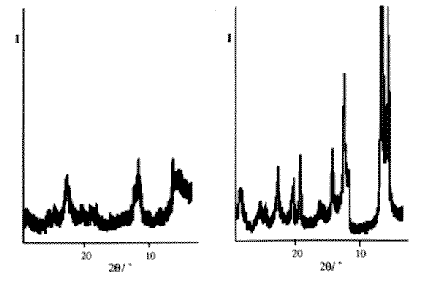
The preparation of oxide and sulphide compounds whose structures are capable of incorporating organic and organometallic compounds between structural layers has led to an explosion of research into intercalation chemistry in recent years. A variety of techniques are used to facilitate this. Whilst some intercalation reactions occur extremely rapidly and without activation, others require the use of thermal or ultrasonic techniques, or even mechanical shaking. Unfortunately, these methods may lead to an undesirable degradation of the product's crystalline structure, leading to difficulties in its characterisation. Once again, this represents a poorly researched area despite the promising findings of initial studies. The intercalation of pyridine and pyridine derivatives into the layered compound a-VO(PO4) proceeds slowly under thermal conditions. The products may be formed with a maximum stoichiometry of a-VO(PO4).py0.85 by exposing the host to the pure pyridine derivative, or alternatively, to a suitable solution of the pyridine in xylene, and heating for long periods. Even after 64 hours, it is only possible to recover an intercalated product corresponding to a-VO(PO4).py0.85 when carried out conventionally in glass ampoules. Although the a-VO(PO4) used in these experiments does not absorb microwaves strongly, the pyridine derivatives do, and are therefore responsible for the heating which occurs to drive the reaction. By placing the a-VO(PO4) host in teflon digestion bombs together with the pyridine, and exposing them to 650Watt microwave radiation, intercalation was found to occur rapidly, with a maximum temperatures and pressures of 200°C/50atm.91 The maximum exposure used was 2x5 minutes. The rate enhancements which were found using this method were relatively large between 102 and 103 times that observed for the conventional synthesis (Table 1). Moreover, the product stoichiometry invariably correspond to that of the fully intercalated material, and the quality of the powder diffraction pattern is enormously enhanced (Figure 1).
Table 1 Intercalation of pyridines in VO(PO)491
Figure 1 Powder diffraction patterns for a) conventionally and b) microwave induced intercalation of pyridine in VO(PO)4
In separate work, the intercalation of aryl tin compounds into the synthetic clay Laponite was shown to result in the formation of tin(IV) oxide pillared clays.92 Conventionally, this may be carried out by mixing the aryl tin compound with the clay in dry ethanol and mechanically shaking the mixture for a week. Following test runs of between 1 and 30 minutes to establish the optimum reaction time, microwaves were used to accomplish an identical reaction by sealing the reactants in a Teflon bomb, and subjecting this to 5x1minute bursts of 700Watt irradiation . Once again, the microwaves appear to have dramatically enhanced the rate at which intercalation occurs (Table 2).
Table 2 Intercalation of aryl tin compounds into laponite clays92
Despite the initially encouraging results, it seems from the 119Sn Mössbauer spectrum of the (Ph3Sn)2O reaction products, that mechanical shaking was superior to the use of the microwaves. In addition, the spectrum indicated the presence of the crystalline aryl tin compound. The authors suggest that the (Ph3Sn)2O in 'excess' of that in the conventional samples is surface-sorbed, rather than truly intercalated. It is also suggested that the aryl tin chlorides have a greater affinity for the clay surface than has the oxide, but undergo hydrolysis with it.
References 91. K. Chatakondu, M.L.H. Green, D.M.P. Mingos & S.M. Reynolds. J. Chem. Soc. Chem. Comm. 1515 (1989). 92. R.C. Ashcroft, et al. Polyhedron11, 1001-1006 (1992).
|
||||||||||||||||||||||||||||||||||||||||||||