|
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Applications of Microwave Heating to Ceramics
© G. Whittaker, 1994 & 2007. This work, or extracts from this work may be reproduced only with the written permission of the author.
Ceramic Materials As with the microwave processing of polymers, the microwave processing of ceramic materials has reached a high degree of maturity. Early work in the field was principally concerned with the removal of solvents from the solid samples,93-96 making use of their dielectric loss properties. It is estimated that below ~5% water content, the microwave drying of materials is more energy efficient than conventional drying methods. Above this level it seems that a hybrid system, incorporating both methods at different stages in the process is most efficient.97 In many cases, microwaves offer superior drying properties to those of thermal techniques, since the power may be more evenly distributed through the sample, leading to more even drying profiles; Once dry, however, it was apparent that many of the compounds were themselves capable of effective dielectric heating.98 Furthermore, rapid progress was made and the strong dielectric loss properties of a large number of oxides,99,100 sulphides,101 and glass ceramics102 was soon recognised. By the use of a screened thermocouple (see Chapter 3) the heating characteristics of over one hundred and fifty compounds were measured,103, 104 representative examples of these data105 are shown in Table 1. It is of particular interest to the author that the metal samples - aluminium and nickel - heat rapidly under these conditions. The rate of heating in these samples is the consequence of a number of factors, and has been discussed in a number of his publications
Table 2Heating characteristics of some solids under microwave irradiation
The single most important and widely investigated application of microwaves in ceramic processing has been in the area of sintering. Initial studies106 were carried out using a 400W microwave tuned waveguide applicator to effect the sintering of alumina and silica rods at >1700oC. Since then, a wide range of materials have been processed in this way, Table 2 summarises a selection of the work which has been published.
Table 2 Sintering of ceramics under microwave irradiation
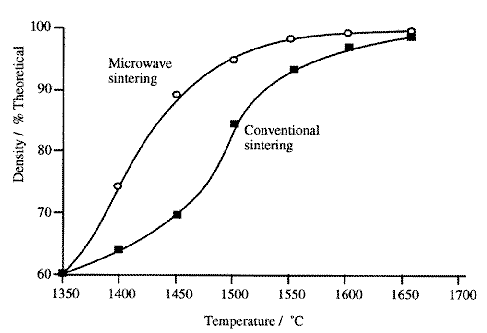
Heating at 2.45GHz may lead to inhomogeneous heating in some volumes of the material during the sintering process. In the microwave sintering of calcium vanadium garnet ferrites, for example, temperatures 100°C above the surface temperature were registered in the sample core.118 A similar result in the microwave treatment of Y-TZP/Y-TZP - Al2O3 samples led to the over-sintering of the sample core.117 When sample homogeneity is of paramount importance, this is clearly undesirable. For this reason, higher frequencies of 28GHz, may be used to smooth out these inhomogeneities.119 Because the wavelength ([lambda]~1cm) in such work is smaller than the sample, it was reasoned that the high field regions leading to inhomogeneous heating would be closer to one another. Ideally, heat conduction from these points through the sample would be rapid enough to give more even sintering. Often, it is not sufficient to heat only the sample, and the importance of using 'caskets' has been studied.120 Containing the sample in a close-fitting material (the casket) with suitable properties allows high temperatures to be reached rapidly, and maintained, and improves the quality of the sintered product. The casket materials must be chemically inert, and may be either microwave absorbing or transparent. The role of transparent materials, for example boron nitride, is simply to act as insulation, retaining the microwave energy which has been converted to heat by the sample. Where the sample has poor dielectric properties at room temperature, such as are observed with alumina, a microwave absorbent material such as zirconia or hafnia may be used to initiate the heating. The advantage of zirconia in particular is that its dielectric heating properties fall off at >=1000oC and allow direct microwave heating of the sample. It has been noted that materials sintered by microwave heating display markedly higher densities for a given reaction temperature and time than do conventionally treated samples, the effect being most evident at low temperatures/sintering densities.113 For a sample of alumina under 700W irradiation, a particular density may be reached at 100oC lower than that required by conventional heating (Figure 1). In other systems, differences as high as 300-400oC have been reported.121 From studies of the rate of densification, it has been suggested that these differences arise from a reduction in the activation energy required for grain boundary diffusion under microwave heating, although a precise mechanism has not yet been fully developed. Despite the technological success of microwave sintering, economic analyses suggest that it does not yet offer energy savings.122 Given that the energy efficiency for conversion of natural gas to microwave power may be as low as 15%, unless microwave sintering can offer improved products or process simplification, it may remain uneconomic for most sintering applications.
Figure 1 Microwave and conventional sintering densities as a function of temperature113
Going beyond sintering, ceramic materials have been successfully melted using microwave heating. During the microwave drying of oxide samples, it was noted that UO2 could be successfully heated to its melting point of >2600°C using microwaves.99 Many other examples of melting have been reported.98, 123-125 Microwave joining has been achieved using a number of ceramic materials. Using a single mode cavity, Palaith et al .126 butt welded two mullite rods together at 1300°C in approximately 10 minutes. Similarly, joining has been reported using either Al2O3 or Si3N4, the latter in an inert atmosphere.127 Interestingly, although it was possible join impure samples (92% and 96% purity respectively) of the ceramics to themselves directly, the pure materials could not be joined without the presence of a thin layer of impure material in the join. This illustrates the importance of impurities in the low temperature microwave heating properties of these compounds (cf. Tan d for 97% pure and 99+% pure alumina in Figure 1.5).
References
93. T. Hirai, I. Tari & T. Ohzuku. Bull. Chem. Soc. Jpn.53, 1477 (1980). 94. P.G. Jolly, Thermodynamics In Australia - Past, Present And Future, Conf On Thermodynamics In Australia - Past, Present And Future, Preprints Brisbane,australia D880509-10, (1988) 95. S.S. Stuchly & M.A. Stuchly. Advances in Drying 53 (1983). 96. R.M. Perkin. J. Separ. Proc. Technol.1, 14 (1979). 97. F.J. Smith. R and D30, 54 (1988). 98. R. Roy, S. Komarneni & L. Yang. Journal Of The American Ceramic Society68, 392-395 (1985). 99. P.A. Haas. Am. Ceram. Soc. Bull.58, 873 (1979). 100. C.E. Holcombe. Am. Ceram. Soc. Bull.62, 1388 (1983). 101. T.T. Chen, J.E. Dutrizac, K.E. Haque, W. Wyslouzil & S. Kashyap.Canadian Metallurgical Quarterly23, 349-351 (1984). 102. J. Macdowell. in American Ceramic Society Bulletin 282-286, ( 1984). 103. J.W. Walkiewicz, G. Kazonich & S.L. McGill. Minerals Metallurgical Processing5, 39 (1988). 104. S.L. McGill, J.W. Walkiewicz & G.A. Smyres, 91st Ann. Meeting Exposition of the Am. Ceram. Soc. April 23-27., Indianapolis, USA, (1989) 105. S.L. McGill & J.W. Walkiewicz. J. Microwave Power Electromagnetic Energy Symp. Summ.22, 175 (1987). 106. A.J. Berteaud & J.C. Badot, J. Microwave Power, (1976) 107. V.K. Varadan, Y. Ma, A. Lakhtakia & V.V. Varadan. MRS Symp. Proc.124, 45 (1988). 108. M.A. Janney & H.D. Kimrey, Ceramic Powder Science Ii, Parts A & B, 1st International Conf On Ceramic Powder Processing Science Orlando,fl D871101-04, (1988) 109. T.T. Meek, R.D. Blake & J.J. Petrovic, 11th Annual Conference On Composites And Advanced Ceramic Materials, 11th Annual Conf On Composites And Advanced Ceramic Materials Cocoa Beach,fl D870118-23, (1987) 110. T.T. Meek, C.E. Holcombe & N. Dykes. J.Mater.Sci.Lett6, 1060 (1987). 111. J.D. Katz, R.D. Blake, J.J. Petrovich & H. Steinburg. MRS Symp. Proc.124, 219 (1988). 112. Y.L. Tian, M.E. Brodwin, H.S. Dewan & D.L. Johnson. MRS Symp. Proc.124, 213 (1988). 113. J. Samuels & J.R. Brandon. J. Mat. Sci.27, 3259-3265 (1992). 114. M.K. Krage. Am. Ceram. Soc. Bull.60, 1232 (1981). 115. H. Kim, H. Kimrey & D. Kim. Journal Of Materials Science Letters10, 742-744 (1991). 116. C. Holcombe & N. Dykes. Journal Of Materials Science26, 3730-3738 (1991). 117. J.L. Shi, Y.L. Tian, B.S. Li, J.K. Guo & D.S. Yan. Sci China Ser A35, 1144-1152 (1992). 118. F. Okada, S. Tashiro & M. Suzuki. Adv. Ceramics15, 201 (1985). 119. B. Swain. Advanced Materials and Processing134, 76 (1989). 120. C.E. Holcombe & N.L. Dykes. J. Mat. Sci. Lett.9, 425 (1990). 121. H.D. Kimrey, M.A. Janney & M.K. Ferber. Ceramic Technology Newsletter20, 3 (1988). 122. S. Das & T.R. Curlee. Amer. Ceram. Soc. Bull.66, 1093 (1987). 123. C. Shibata & H. Tamai. J. Microwave Power and Electromagnetic Energy25, 81 (1990). 124. R.B. James, P.R. Bolton, R.A. Alvarez, W.H. Christie & R.E. Valiga.J. Appl. Phys.64, 3243 (1988). 125. S. Komenarni & R. Roy. Materials Lett.4, 107 (1986). 126. D. Palaith, R. Silberglitt, C.C.M. Wu, R. Kleiner & E.L. Libelo.MRS Symp. Proc.124, 255 (1988). 127. H. Fukushima, T. Yamanaka & M. Matsui. MRS Symp. Proc.124, 267 (1988).
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||